Tissue Technology
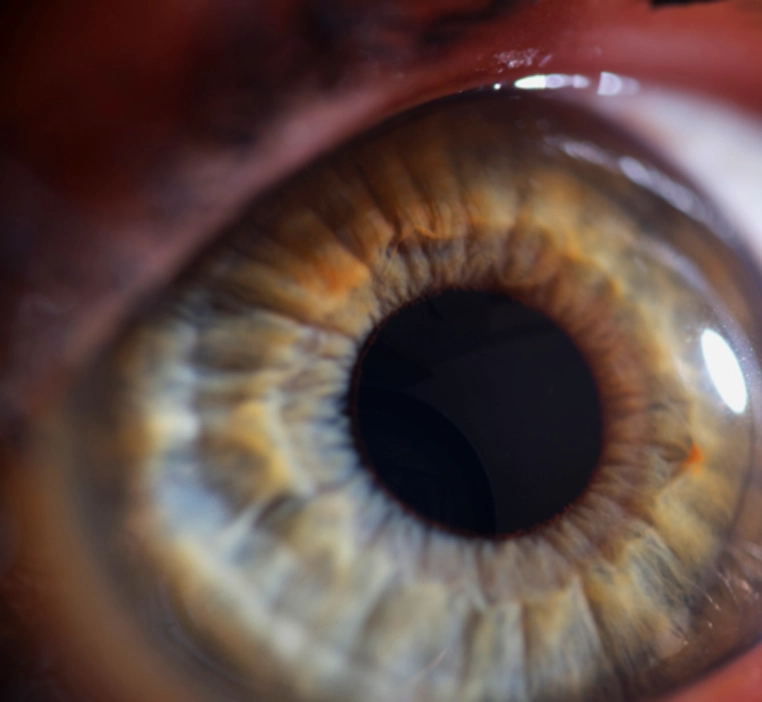
Tissue technology for the cornea represents an innovative approach wherein healthy corneal tissue from a donor is carefully integrated into the existing cornea of a recipient to enhance its structure and function. This technique is especially valuable, given that the cornea plays a critical role in focusing incoming light onto the eye's interior. By precisely shaping and grafting this tissue, tissue technology aims to restore optical clarity and improve vision quality. This procedure holds significant potential for individuals affected by various visual disorders, including presbyopia, hyperopia, myopia, astigmatism, and keratoconus, providing them with a renewed opportunity to achieve better vision and an improved quality of life.